This page is aimed at the novice, it may be pretty boring to others.. I have no formal qualifications in refrigeration, physics or tea making. The following information could be inaccurate or just plain wrong.
*To save space I'll mainly use the term absorption and absorber even though sometimes this could also be adsorption and adsorber*
There are two main types of absorption cooling cycle. One is a continuous cycle where heat can be applied continuously and the cold part of the system always stays cold. This is the cycle used in gas/kero fridges. The other system is an intermittent cycle where there are two distinct phases. There is a generation phase where the generator (which is the absorber later) is heated and the receiver( which is the evaporator later) is at close to ambient temperature and a cooling phase where the absorber is near ambient and the evaporator is cold. Each system has its positives and negative.
So how does an absorption fridge work? First some basic kitchen physics.
After a long day of kitchen table top experiments, Dave and I need a tea break to sit down and figure out why is it - that in theory absorption fridges are simple but in practice they're not. Why, in theory - theory and practice are the same but in practice they're not - and other deep stuff like that.
So I put some water in the kettle and put it on the stove and turn it on. If we were to measure the temperature of the water, we would notice a steady rise in temperature until the water started to boil, after that - the temperature would stay the same (assuming the water is pure and some other parameters are constant). Before the water boils the energy (heat) from the stove raises the temperature (sensible heat), once water boils the energy goes into changing the phase of the water from liquid to gas (latent heat). If I forget to turn to heat off, the water will stay at the same temperature until all of it has changed to steam. The steam at the surface will be the same temperature as the water. If the pressure in the kettle is one standard atmosphere the water will boil at 100 deg C, if the pressure is higher - so is the boiling point, likewise if the pressure is lower - so is the boiling point.
There is an other way to boil the water, one which is not useful for making tea but is useful for refrigeration. We can boil water by lowering the pressure until the boiling point is lower than room temperature. Then the water will boil and absorbs heat from its surroundings, ie. it becomes cold. This type of heat absorption is the basis for most refrigeration systems. If we lower the pressure enough the water will cool itself down to freezing point and simulatously boil and freeze. This happens at around one two hundredth of an atmosphere. Other liquids have similar behavior but at different temperatures and pressures, and many can remain as liquids below 0 deg C, this is essential if we want to build a freezer.
Kitchen physics is hungry as well as thirsty work. So after making a clear space amongst the debris, I chop some vegies and put them in a steamer. Some water goes into the bottom and I heat it to boiling point, the boiling water absorbs energy from the stove, the steam condenses on the surfaces of the pot and food, in condensing it releases the energy (latent heat) and heats all the surfaces to 100 deg C. Excess water runs back the bottom. All the air is displaced by steam and heat continues to be moved from the bottom of the pot to the contents by the phase changes in the water - from water to steam and back again. If, assuming all the air and other gasses had been expelled ,we now seal the pot with a gas tight seal (and the pot is strong enough). Now the normal boiling point is no longer relevant. The steam will continue to transport heat from the bottom to the contents at wide range of temperatures, we have constructed a heat pipe. Ok lets forget about food for a while and just consider a simpler heat pipe - say a piece of vertical tube sealed with water in the bottom and all the air removed. Most people should be able to visualize that heating the bottom will cause the water to boil and the steam condensing at the top will heat it. What may be more difficult is visualizing that cooling the top will also cool the bottom. If we cool the top steam will condense lowering the pressure inside the tube such that the water at the bottom will boil until it has cooled down to match the temperature at the top. We now know how to move heat around using latent heat, next we will make a heat pump.
Before we talk about absorption let's talk about a simple compressor fridge.
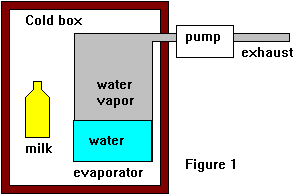
In the above text I've explained how we can cool water by boiling in at low pressure, we've also seen how we can heat things by condensing vapor into liquid. So let's make a simple cooler to keep the milk for the tea fresh. Let's make a small thermally insulated box and inside it place a flask of water (the evaporator) with a pipe running from the top of the flask to a pump outside the box.. We run the pump, lower pressure, boil the water and lower the temperature. We might call this an intermittent compressor fridge because we need to stop it once in a while to refill the water.
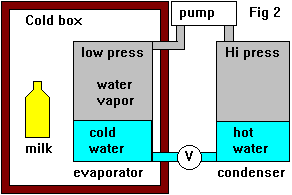 Since this is a hassle we fit another container (condenser) to the outlet from the pump and condense the steam back into water, this container will become hotter due to the latent heat of the condensation. The condenser is at a higher pressure than the evaporator. We now have a heat pump of sorts. Finally we run a pipe back into the evaporator via a valve which maintains a pressure difference between the evaporator and condenser, this might just be a narrow tube. Now we have a continuous cycle, this is how most fridges work. Usually the refrigerant won't be water and the pressures in the system will be much higher. The containers will usually be tubes to improve heat flow but the principle is the same.
Since this is a hassle we fit another container (condenser) to the outlet from the pump and condense the steam back into water, this container will become hotter due to the latent heat of the condensation. The condenser is at a higher pressure than the evaporator. We now have a heat pump of sorts. Finally we run a pipe back into the evaporator via a valve which maintains a pressure difference between the evaporator and condenser, this might just be a narrow tube. Now we have a continuous cycle, this is how most fridges work. Usually the refrigerant won't be water and the pressures in the system will be much higher. The containers will usually be tubes to improve heat flow but the principle is the same.And now what you came here for, the absorption stuff.
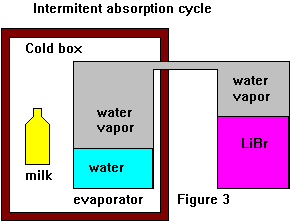 To build an intermittent cycle fridge we don't have to use a mechanical pump to lower the pressure in the evaporator. Just say we had something that absorbed the water vapor, some chemical with a hunger for water that would grab enough water molecules the keep the vapor pressure low enough to allow the water to boil at low temperatures. Such chemicals exist, one of them is a salt called Lithium Bromide(LiBr). If we replace the pump and condenser with a container of LiBr and remove the air - presto we have absorption cooling (in theory anyway). This cooling phase is the evaporation/absorption part of the cycle. The system would continue to cool till either we run out of water or the LiBr has absorbed all the water it can. What now? We remove the contraption from the box and heat the LiBr, it changes from being an absorber to being a generator. We cool (as best we can) what was the evaporator and it becomes the condenser/receiver. This cooling may just be exposure to room temperature air or a dip into cool water. During this part of the cycle water is released by the LiBr and condenses back into the receiver ready for another cooling cycle. If we can heat the LiBr enough we can remove all the water, but at lower generator temperatures we must be content with only removing some of the water. Note that the pressure is governed by the receiver temperature. At the generator temperatures we can expect from a passive solar collector, we might only change the concentration of the LiBr solution by 5% to 10 %. Unfortunately LiBr is too expensive to build a practical intermittent system and as it uses water as the refrigerant it can't be used to make a freezer, also the working pressures are very low and hard to maintain. intermittent cycle absorption fridges need far more absorber and refrigerant than continuous cycle ones. For a more practical cycle see the icyball patent. This uses an ammonia/water cycle, ammonia is the refrigerant and water the absorber, the system works at much higher pressures. Also see - Solar Powered Refrigeration by means of an Ammonia/water Intermittent Absorption Cycle. There are more complicated intermittent cycle systems, for example we might spilt the receiver into two allowing the condensation to occurs outside of the fridge and the evaporation to occurs inside. One might also build a pseudo continuous cycle by using multiple generator/absorbers. This could be useful in an adsorption system where the adsorber is a solid and unsuitable for a continuous cycle.
To build an intermittent cycle fridge we don't have to use a mechanical pump to lower the pressure in the evaporator. Just say we had something that absorbed the water vapor, some chemical with a hunger for water that would grab enough water molecules the keep the vapor pressure low enough to allow the water to boil at low temperatures. Such chemicals exist, one of them is a salt called Lithium Bromide(LiBr). If we replace the pump and condenser with a container of LiBr and remove the air - presto we have absorption cooling (in theory anyway). This cooling phase is the evaporation/absorption part of the cycle. The system would continue to cool till either we run out of water or the LiBr has absorbed all the water it can. What now? We remove the contraption from the box and heat the LiBr, it changes from being an absorber to being a generator. We cool (as best we can) what was the evaporator and it becomes the condenser/receiver. This cooling may just be exposure to room temperature air or a dip into cool water. During this part of the cycle water is released by the LiBr and condenses back into the receiver ready for another cooling cycle. If we can heat the LiBr enough we can remove all the water, but at lower generator temperatures we must be content with only removing some of the water. Note that the pressure is governed by the receiver temperature. At the generator temperatures we can expect from a passive solar collector, we might only change the concentration of the LiBr solution by 5% to 10 %. Unfortunately LiBr is too expensive to build a practical intermittent system and as it uses water as the refrigerant it can't be used to make a freezer, also the working pressures are very low and hard to maintain. intermittent cycle absorption fridges need far more absorber and refrigerant than continuous cycle ones. For a more practical cycle see the icyball patent. This uses an ammonia/water cycle, ammonia is the refrigerant and water the absorber, the system works at much higher pressures. Also see - Solar Powered Refrigeration by means of an Ammonia/water Intermittent Absorption Cycle. There are more complicated intermittent cycle systems, for example we might spilt the receiver into two allowing the condensation to occurs outside of the fridge and the evaporation to occurs inside. One might also build a pseudo continuous cycle by using multiple generator/absorbers. This could be useful in an adsorption system where the adsorber is a solid and unsuitable for a continuous cycle. Now let look at doing a continuous cycle absorption fridge.
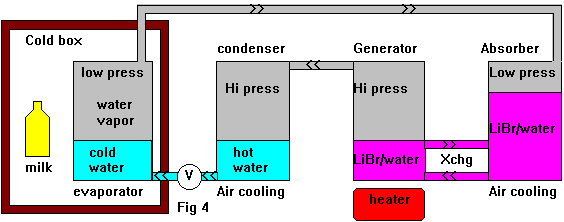 In the continuous cycle fridge we discussed above we used a mechanical pump to maintain a pressure difference across a pressure reducing valve with the aim of boiling a liquid as it passed from the high pressure to low pressure parts of the system, we want to do something similar now using absorption techniques. We arrange two containers of LiBr/water solution so that the solution can circulate between to two, probably using a pair of tubes. We heat one container (the generator) and it generates gas (water vapour) - raising the pressure in the container with respect to the cold one. In the process the solution becomes more concentrated. The pressure increase will also force some solution across to the other container causing a difference in the liquid levels. We can run a pipe from the top of the generator taking the hot gas into a condenser to liquify it. The liquid then passes through a pressure reducing valve and boils in the low pressure side of the system. This gives us the desired cooling effect. The low pressure gas then travels into the cooler container (absorber) where it is absorbed by the LiBr solution. The LiBr is circulated from one container to the other, absorbing gas on the cool side and releasing it on the hot side. The transfer of heat from one container to the other by the LiBr solution is undesirable and can be reduced with the addition of a counter flow heat exchanger. A heat exchanger between the warm water entering the evaporator and the cold gas leaving it may also improve efficiency.
In the continuous cycle fridge we discussed above we used a mechanical pump to maintain a pressure difference across a pressure reducing valve with the aim of boiling a liquid as it passed from the high pressure to low pressure parts of the system, we want to do something similar now using absorption techniques. We arrange two containers of LiBr/water solution so that the solution can circulate between to two, probably using a pair of tubes. We heat one container (the generator) and it generates gas (water vapour) - raising the pressure in the container with respect to the cold one. In the process the solution becomes more concentrated. The pressure increase will also force some solution across to the other container causing a difference in the liquid levels. We can run a pipe from the top of the generator taking the hot gas into a condenser to liquify it. The liquid then passes through a pressure reducing valve and boils in the low pressure side of the system. This gives us the desired cooling effect. The low pressure gas then travels into the cooler container (absorber) where it is absorbed by the LiBr solution. The LiBr is circulated from one container to the other, absorbing gas on the cool side and releasing it on the hot side. The transfer of heat from one container to the other by the LiBr solution is undesirable and can be reduced with the addition of a counter flow heat exchanger. A heat exchanger between the warm water entering the evaporator and the cold gas leaving it may also improve efficiency. The diagrams above are simplified and may look nothing like a real fridge. A real fridge would probably use tubes and fins in many places. The size of the various parts could vary considerably along with their shape and placement. I hope this has been useful just the same.
Absorber/refrigerant pairs.
There a zillions of possible absorber/refrigerant pairs, many are still untested (or undiscovered) - none that I know of are perfect. The perfect pair would be cheap, nontoxic, non corrosive, environmentally safe, have a convenient working pressure, low absorber specific heat, high refrigerant latent heat and so forth. A continuous cycle requires a liquid absorber, an intermittent cycle can use either a liquid absorber or a solid adsorber.
The working pressure is governed by the refrigerant, the pressure will be what ever is required to make the refrigerant boil at the desired evaporator temperature and will be higher in the generation phase for intermittent cycle or in the generator in a continuous cycle. The ideal working pressure is probably lightly above atmospheric pressure. This is because a slight leak of refrigerant out of the system will degrade performance less than a leak of air into the system.
Some working pairs include, Ammonia/water, Ammonia/Calcium chloride(solid), Lithium bromide/water, Zeolite/water and Activated charcoal/methanol.
- Water makes a poor refrigerant where low temperatures are required. You obviously can't make a freezer using it because the refrigerant will freeze before your ice-cream does. Even at slightly higher temperature (say 4C) its vapour pressure is still very low. However it may be suitable for air-conditioners but even here the low pressure can be a problem. Well at least its cheap!
- Ammonia works at much higher pressures around 5-12 atm, this has its problems too. When used in a continuous cycle, you get a big pressure difference between the absorber and generator, this requires a pump to make it work. However adding hydrogen as a "capping gas" fixes this problem , I think this means you have a mixture of hydrogen and ammonia in what is labelled "low pressure vapour" in fig 4. The reactions are based on the partial pressures of ammonia not the total pressure of the mixture - so we can increase the total pressure to reduce the liquid level difference between the generator and absorber so that a pump is no longer needed. When Ammonia/water is used as a generator - water is released with the ammonia. This degrades performance and requires that intermittent systems be "reset" often by pouring the excess water back where it belongs. Ammonia is poisonous and corrosive.
- Methanol, higher pressures than water but still pretty low, a low latent heat, toxic, inflamible (explosive?), often contaminated with water. It is unstable at high temperatures when catalysts are present.
Calcium chloride when adsorbing ammonia increases its volume about four fold. The CaCl has to be processed into granules so the ammonia (gas) can penetrate. - Lithium bromides main vice is that its very expensive.
- Activated charcoal is a better thermal insulator than we would like. It may also already contain trapped chemicals you have to get rid of before you can use it.
Is it worth while?
Comparing absorption and electric (compressor based) fridges is not a simple matter. In my opinion the photo-voltaic (PV) electric fridge is better in most remote power situations. While the absorption fridge is simple in concept one must consider things like backup systems for cloudy days (eg. gas boost), reliability, portability and so on. The niches for solar absorption may include :-
1 - Developing countries that don't have the capital to import PV panels.
2 - Alternated lifestyle people who like to tinker.
3 - Large scale cooling where scaling factors may favour solar absorption systems.
and so forth.
One should note that an efficient PV electric fridge is unlikely to look like a normal domestic fridge. It would probably be a top opening chest with motor, compressor and condenser move away from the cold box - possibly outside the house. It should (in my humble opinion) use ice storage for overnight cooling and not batteries.